 User Guide — Electronic Case Reporting
User Guide — Electronic Case Reporting
Per the CMS, electronic case reporting is required for providers who participate in a quality program.
There are two components to the feature:
-
eCR Now App – A Fast Healthcare Interoperability Resources (FHIR) App integrated in athenaOne. It sends electronic case reports (eCR) to public health agencies at the local or state level.
-
Case Report Documents Report – Report that shows reportability response documents received from public health agencies in a specified time period.
Case reporting
When a provider sees a patient who could have a contagious disease that is deemed reportable for public health reasons, the provider must submit a case report to the appropriate public health agencies.
This is how providers currently submit a case report:
-
By phone
-
By fax
-
By logging in to the portal for the state or local registry
Electronic case reporting (eCR)
With the outbreak of COVID, it was very difficult for the federal government to report the number of cases that were diagnosed in each state. Of the diagnosed cases, it was also difficult to report the number of active and resolved cases.
The Centers for Disease Control and Prevention (CDC) and the federal government developed an app for electronic case reporting that companies such as athenahealth can integrate with their electronic health records (EHR) system.
That solution is the eCR Now Fast Healthcare Interoperability Resources (FHIR) App. We integrated this app in athenaOne.
CMS requires electronic case reporting
Please note that per the Centers for Medicare & Medicaid Services (CMS), the Electronic Case Reporting (eCR) measure is being suppressed from scoring calculations for Performance Year (PY) 2025.
The Centers for Medicare & Medicaid Services (CMS) now requires electronic case reporting to meet the following public health objectives:
-
Merit-based Incentive Payment System (MIPS) Promoting Interoperability Program – This requirement impacts providers who use athenaClinicals.
For details, see https://qpp.cms.gov/docs/pi_specifications/Measure%20Specifications/2023MIPSPIMeasuresElectronicCaseReporting.pdf.
-
Medicare Promoting Interoperability (PI) Program – This requirement impacts providers who use athenaClinicals for Hospital & Health Systems, starting in payment year (PY) 2022.
For details, see https://www.cms.gov/medicare/regulations-guidance/promoting-interoperability-programs.
By using eCR Now in athenaOne, you meet both requirements.
We will enable eCR Now for all providers and contexts. If your practice does not participate in a quality program and wants eCR Now, you can request it.
Electronic case reporting is required by CMS for you to receive a MIPS PI category score, which contributes to an eligible clinician's overall score and ultimately determines the MIPS payment adjustment. If you choose to opt out of this functionality, it could impact your MIPS reimbursement. If your practice would like to opt out of electronic case reporting, contact the CSC and ask for electronic case reporting to be turned off.
This feature has been rolled out in phases across states over the past two years. Some states are not yet ready for eCR. You will receive an athenaNetwork post from athenahealth once the feature becomes available for your organization.
In athenaOne, eCR Now is an optional feature. If you want to continue to phone or fax cases, you can choose not to enable eCR Now. However, we encourage you to use eCR Now because it:
-
Is the best practice
-
Saves time
-
Reduces your work
-
Reports cases more quickly than a manual process
Important
To achieve incentive points for PI programs, you must use eCR Now to submit electronic case reports.
eCR Now runs in the background and it performs the following actions:
-
athenaOne notifies eCR Now when a user performs one of the following actions:
-
Checks in a patient for an encounter
-
Creates a hospital stay for a patient
-
Creates an order group for diagnoses and medications
-
-
eCR Now checks the following information that is documented for an encounter or hospital stay:
-
Problem list
-
Diagnosis
-
Lab orders
-
Medication prescriptions
-
Lab results
-
-
eCR Now then:
-
Compares the information it gathers to a list of reportable events
-
Checks for information throughout the life of the encounter or hospital stay on this schedule:
-
One hour after an action in step 1 begins. The objective of this check is to determine if there are any reportable events that need to be immediately reported. This is a narrow check that looks only for a subset of reportable events.
-
Nightly until the encounter or hospital stay reaches the final status. This is a thorough check that looks for all reportable events.
-
72 hours after the encounter or hospital stay reaches the final status.
Important
This is the final check. It captures lab results received after step 3b ii.Lab results that are received more than 72 hours after the encounter or stay is closed will not be checked for reportable events. If a document received after this time period contains a reportable event, you are expected to manually report to the appropriate public health agency (PHA).
-
-
Checks for reportable events when an encounter is amended. Any reportable events that are created from adding clinical data via the amendment workflow will be electronically reported to the appropriate PHA.
-
Stops checking for reportable events in encounters that are still open after 3 days and inpatient stays that are open after 7 days. Clinical data added after these thresholds will need to be manually checked for reportable events and reported to the appropriate PHA.
-
-
If eCR Now:
-
Detects a reportable event in step 3a – eCR Now creates and sends an electronic initial case report (eICR) to the appropriate PHA.
An initial case report is one that the public health registry will review to determine if the case is, indeed, reportable or not.
Go to step 5.
-
Does not detect a reportable event in step 3a – eCR Now stops checking the encounter or hospital stay.
-
-
The public health agency acknowledges receipt of the case report by sending a reportability response to the patient chart.
This document is:
-
In CLOSED status so no action is required.
-
Listed in the Find tab under Clinical Document.
The new sub-category is Case report. The title is clinical document – Case Report:
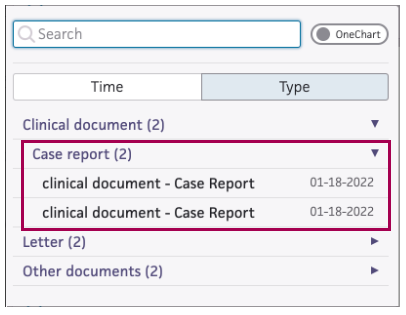
-
Note: State PHAs may reach out to you as they are comparing what is being reported manually and what is being reported electronically. The PHAs will notify you when you can stop manually reporting.
Our eCR Now application is monitored by an internal team for outages or issues. We have alerts and dashboards in place to ensure the product is healthy and functioning as expected at all times. In the unlikely event of a catastrophic outage event (prolonged outage of more than 48 hours) we will communicate guidance.
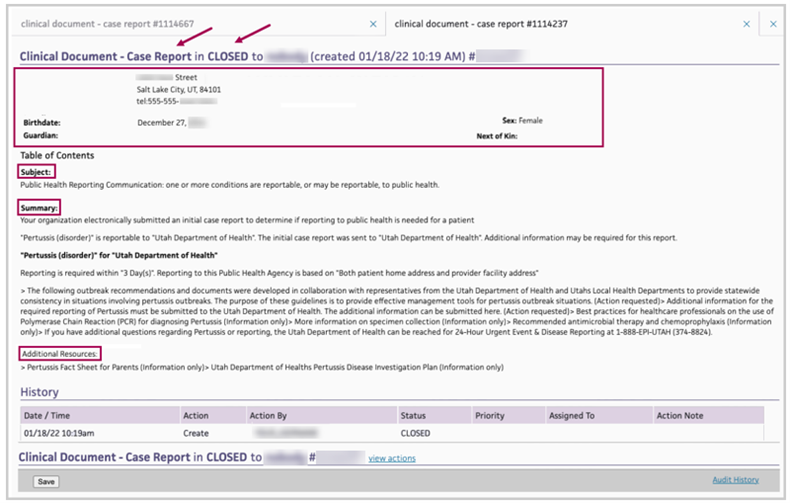
This is an example of a reportability response from the Utah Department of Health:
-
It acknowledges receipt of an eCR for a female patient who has pertussis.
-
The sections are Subject, Summary, and Additional Resources.
-
The document goes to the patient chart in CLOSED status.
-
No further action is required on your part.
Case Report Documents is a report that lists reportability response documents received from public health agencies over a period of time that you specify.
The columns in the Case Report Documents report are:
-
Patient Full Name
-
Patient ID
-
Provider Full Name
-
Provider NPI
-
Document ID – Click the ID number to go to the document in the patient chart.
-
Document Created Date
To add the report to your Report Library:
-
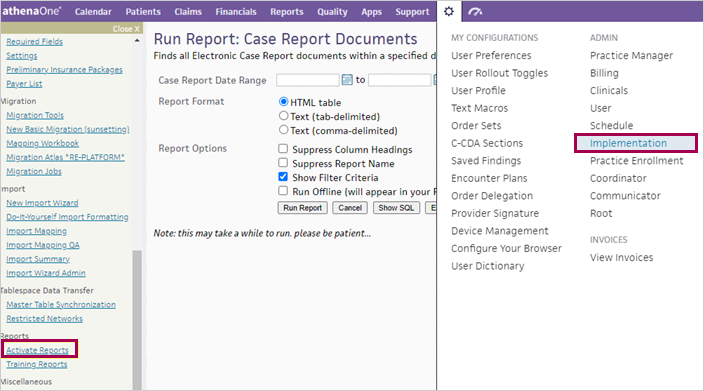
On the Main Menu, click the Settings icon
 .
. -
Under ADMIN, click Implementation.
-
In the Task Bar, under PRACTICE LINKS — Reports, click Activate Reports.
-
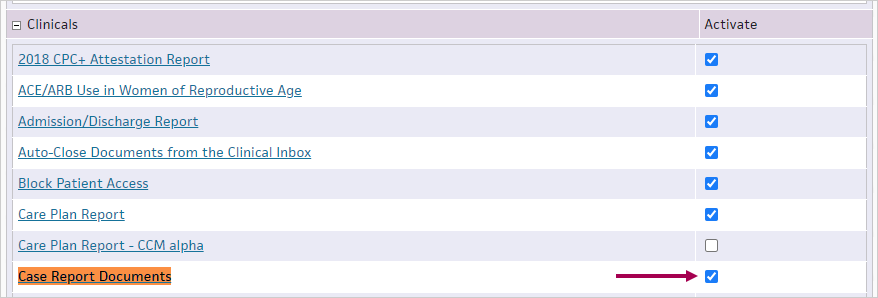
Search for Case Report Documents and check the box next to it.
-
At the bottom of the page, click Save.
Case Report Documents is added to the Clinicals tab in the Report Library.
- Display the Report Library: On the Main Menu, click Reports. Under General, click Report Library.
- Click the Clinicals tab.
- Click run next to Case Report Documents in the Standard Reports section of the tab.
The Run Report: Case Report Documents page appears. - Case Report Date Range — Enter the start and end dates for the range of reportability response documents that you want to include in the report, or select a date range from the menu.
- Report Format — Select the report format:
- HTML table — Display the report results on your screen.
- Text (tab-delimited) — Export the report results to a .csv file in tab-delimited format.
- Text (comma-delimited) — Export the report results to a .csv file in comma-delimited format.
- Report Options — Select the desired options:
- Suppress Column Headings — Select this option to remove column headings from the report results.
- Suppress Report Name — Select this option to remove the report name from the report results.
- Show Filter Criteria — Select this option to include your selected filter criteria in the report results.
- Run Offline (will appear in your Report Inbox tomorrow morning) — Select this option for very long reports. Reports that are run offline appear in your Report Inbox the morning after the request.
- Click Run Report.