Implantable Device List Report
athenaOne for Hospitals & Health Systems
The Implantable Device List report lists implant devices by the implant's reference (REF) number, lot (LOT) number, and expiration date range.
Display the Report Library: On the Main Menu, click Reports. Under General, click Report Library. On the Clinicals tab, click run next to the Implantable Device List report in the Standard Reports section.
To access reports on the Clinicals tab of the Report Library, you must have the Clinicals user permission and the Report: Report Library: Clinicals permission. The Report: Report Library: Clinicals permission is included in the following roles:
- Practice Superuser role
- Report Reader role
Note: To activate the Implantable Device List report, use the Activate Reports page.
- Display the Report Library: On the Main Menu, click Reports. Under General, click Report Library.
- On the Clinicals tab, click run next to the Implantable Device List report in the Standard Reports section.
Select the details to include in the reports
- Manufacture Date Range — Click the first calendar icon
 to select a "from" date. Click the second calendar icon
to select a "from" date. Click the second calendar icon  to select a "to" date.
to select a "to" date.
Note: You can also click the menu to select a time frame. - Implant Date Range — Click the first calendar icon
 to select a "from" date. Click the second calendar icon
to select a "from" date. Click the second calendar icon  to select a "to" date.
to select a "to" date.
Note: You can also click the menu to select a time frame. - Expiration Date Range — Click the first calendar icon
 to select a "from" date. Click the second calendar icon
to select a "from" date. Click the second calendar icon  to select a "to" date.
to select a "to" date.
Note: You can also click the menu to select a time frame. - Exclude devices with no recorded manufacture date? — Select this option to exclude devices with no manufacture date from the report.
- Exclude devices with no recorded implant date? — Select this option to exclude devices with no implant date from the report.
- Exclude Inactive Implants? — Select this option to exclude inactive implants from the report.
- Device Identifier (DI). Enter '*' for all devices. — Enter the device identifier, or enter '*' for all devices.
- LOT. Enter '*' for all devices. — Enter the LOT number, or enter '*' for all devices.
- REF number. Enter '*' for all devices. — Enter the REF number, or enter '*' for all devices.
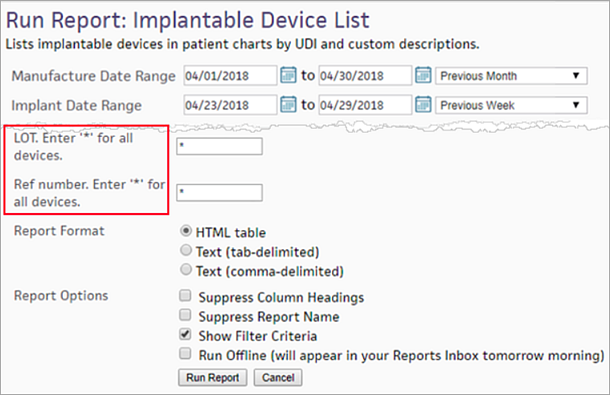
Select the report format and run the report
- Report Format — Select your report format:
- HTML table
- Text (tab-delimited)
- Text (comma-delimited)
- Report Options — Select report options:
- Suppress Column Headings
- Suppress Report Name
- Show Filter Criteria
- Run Offline (will appear in your Reports Inbox tomorrow morning)
- Click Run Report.
The report includes the REF, Expiration Date, and LOT columns.